That's right! Visually, the glass pane is solid, but in reality it is a viscous liquid.

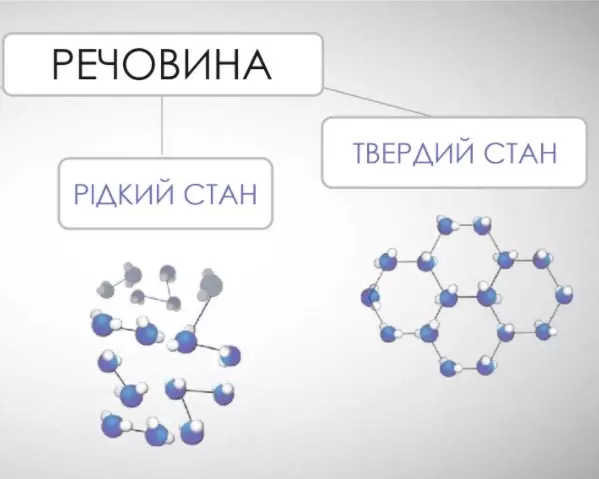
Why? Because glass is an amorphous substance, meaning it has no crystalline structure.
It does not crystallize, but hardens, forming a perfectly smooth surface.

How exactly?
The main component of glass is silica (quartz) molecules, which do not form an ordered structure. In production, glass is cooled so quickly that when it changes from a liquid to a so-called “solid” state, the molecules do not have time to take on the ordered crystal structure characteristic of solids. This is why glass is still liquid even when it is visually solid.

The example of window frames in old houses that have stood for many decades will help to make sure of this. The glass in such frames is noticeably thinner at the top than at the bottom. This is due to the fact that some of the glass has flowed down over the years, and as a result, in such houses the windows rattle in the frames, and cracks form, because the top is much thinner than the original gap in the frame.
Thus, glass is an extremely viscous supercooled liquid that reaches a solid glassy state when it cools at a rate sufficient to prevent crystallization. At the same time, glass retains the basic properties of a liquid even in its apparently solid state.